Medical device for preventing intra-uterine synechiae
Context
Causes
- Aggression of the endometrial mucous
- Intra-uterine surgical intervention
- Infection contracted during a surgical intervention (IUD placement), or due to a pathology
Definition
- Partial or total adhesion of the uterine lining, also known as Asherman Syndrome
- Formation of progressively calcifying fibrous bands in the uterine cavity
Consequences
- Pelvic pain
- Menstrual cycle problems
- Mechanical infertility due to faulty implantation of fertilized egg
- Obstetric complications

Technology
Antisyn System is an intra-uterine medical device offering a temporary anti-adhesion effect. It is designed for use in cases of fertility failure in patients who have undergone endo-uterine procedures.
*MD: medical device
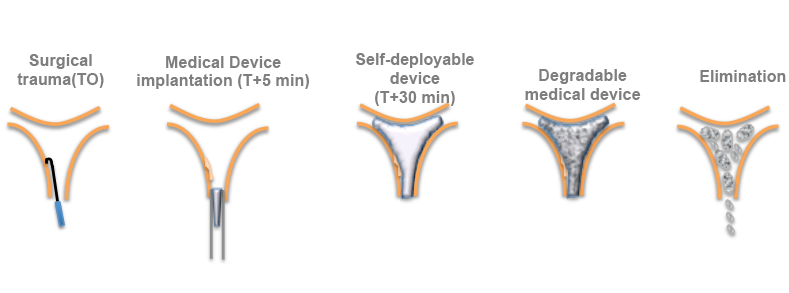
Markets
There are no medical devices of this type on the market, and this device meets the needs of clinical practitioners perfectly. Antisyn System is situated at the cross-roads of applied biomaterials research and clinical gynecological applications.
The expertise developed by Antisyn System integrates the properties of synthesized polymers that meet the expectations of gynecological surgeons and obstetricians for this type of medical device.
Benefits
Antisyn System is a medical device that is sterilizable, self-deployable, anti-adhesive, and bioabsorbable.
This medical device is comprised of a biocompatible polymer.
Ease of implantation with adapted deployment and inflation properties. The medical device is provided in “dry form”, presenting dimensions that enable easy placement from the uterine cervix (diameter of about 5 - 8 mm). Once in the uterine cavity, it absorbs biological liquid and adapts to the cavity shape as it deploys.
The medical device is painless for the patient and has no effect on biological fluid passage.
This physical barrier keeps the uterine walls separated during the postoperative period (5 to 7 days), without affecting proper endometrium regeneration.
Anti-adherence properties limit the proliferation of fibroblasts originating processes.
The polymer used in the medical device is resorbed and eliminated by natural means 5 to 7 days following surgery.
Elimination of the medical device is painless for the patient, and has no effect on menstruation or reproduction.
Patents
Patent filed
Possibility for exclusive license

Team
Antisyn System comprises a multi-disciplinary team including chemistry researchers and hospital practitioners:

Pr Xavier Garric
Antisyn System project lead
Elaboration and follow-up on implementation of specifications, project management, and participation in various design phases of the medical device.
- PhD in Pharmacy
- PhD in Biological and Chemical Sciences for Health
- Certified research director
- University professor
- Co-director of the Chemistry Department, Université de Montpellier

Salomé Leprince
.
Elaboration of specifications, synthesis and characterization of polymers, implementation, pre-clinical trials, and scale-up.
- Master's degree in Health Engineering, “Biomaterials and Medical Devices”
- PhD student in Biological and Chemical Sciences for Health
- Maturation Engineer (SATT AxLR)

Dr Stéphanie Huberlant
.
Elaboration of specifications and evaluation of medical device’s therapeutic potential.
- PhD student in Biological and Chemical Sciences for Health
- Diploma in Specialized Complementary Studies: Reproductive Medicine
- Gynecologist-Obstetrician
- Hospital practitioner in the Gynecology Department, Nîmes University Hospital

Cédric Paniagua
.
Synthesis and characterization of polymers, scale-up.
- Technical University Diploma in Chemistry, Université de Montpellier
- Assistant CNRS Engineer
- In charge of synthesis and quality of the Polymer bench of the Synbio3 Technology Platform

Pr Jean Coudane
.
Synthesis and characterization of polymers, scale-up.
- Associate University Professor in Chemistry
- PhD in Macromolecular Chemistry
- Distinguished Professor, Université de Montpellier
- In charge of the “Artificial Biopolymers” department at IBMM
Pr Vincent Letouzey
.
Elaboration of specifications and evaluation of medical device’s therapeutic potential.
- Gynecologist-Obstetrician
- PhD in Biological and Chemical Sciences for Health
- Certified research director
- Professor (PU-PH) in the Gynecology Department, Nîmes University Hospital










